The Reactive Steel Coating Thickness Challenge
Reactive steels—those containing silicon and phosphorus concentrations that catalyze accelerated zinc-iron alloying reactions—routinely produce coating thicknesses substantially exceeding specification minimums and customer expectations. While thicker coatings inherently provide extended corrosion protection proportional to increased thickness, several practical concerns motivate efforts to moderate excessive coating growth. Understanding the metallurgical mechanisms driving reactive coating formation, available process controls, and their limitations enables galvanizers to optimize coating thickness when reactive steel is unavoidable while managing realistic expectations about achievable results.
Why Coating Thickness Control Matters
Despite the corrosion protection advantages of thick coatings, several legitimate concerns justify attempting to limit coating growth on reactive steels:
Coating Brittleness and Flaking
Excessively thick zinc-iron intermetallic layers exhibit inherent brittleness due to their crystalline structure and ordered atomic arrangements. The zeta (ζ) phase—the predominant layer in reactive steel coatings—demonstrates particular brittleness when layer thickness exceeds approximately 150-200 micrometers (6-8 mils).
Mechanical Vulnerability:
Thick intermetallic coatings lack the ductility of pure zinc eta layer, making them susceptible to mechanical damage during:
Handling and Transportation: Articles experience impacts, abrasion, and flexure during lifting, transport, and storage. Brittle thick coatings can crack or spall from these routine handling stresses.
Assembly Operations: Bolting, fitting, and erection activities impose localized stresses that may exceed brittle coating fracture thresholds, causing coating fragments to detach.
Thermal Cycling: Differential thermal expansion between steel substrate and thick intermetallic layers during temperature changes can generate interfacial stresses causing coating delamination.
Service Loading: Structural members experiencing flexure, vibration, or impact during service may shed coating fragments from thick brittle layers unable to accommodate substrate deformation.
Flaking Consequences:
Coating loss through flaking exposes underlying steel to atmospheric corrosion, potentially initiating deterioration despite initially excessive coating thickness. Localized bare areas corrode preferentially, creating aesthetic defects and potentially reducing effective service life below what properly adhered thinner coatings would provide.
Appearance Variations
Reactive steels produce characteristic appearance differences from normal galvanizing:
Matte Gray Finish:
Extensive zinc-iron alloying consumes most or all of the bright, metallic pure zinc eta layer. The exposed zeta phase intermetallic displays matte gray coloration lacking the characteristic bright spangled or non-spangled metallic appearance associated with galvanizing.
Non-Uniform Appearance:
Articles with varying section thicknesses or mixed steel chemistries exhibit coating thickness variations producing patchwork appearance—some areas showing bright metallic finish while adjacent zones display dull gray, creating visually jarring inconsistency.
Rough Surface Texture:
Pronounced intermetallic crystal growth creates rough, irregular surface texture contrasting with the relatively smooth texture of normal coatings. This roughness affects tactile quality and can complicate fit-up of precision assemblies.
Aesthetic Acceptability:
While appearance concerns don't affect corrosion protection functionality, many applications—architectural metalwork, consumer products, equipment housings—require consistent, attractive finishes. The distinctive appearance of reactive steel coatings may violate aesthetic specifications despite meeting protective performance requirements.
Premature Brown Staining
Zinc-iron intermetallic alloys exposed at or near the coating surface exhibit different atmospheric weathering behavior compared to pure zinc:
Accelerated Patina Formation:
Intermetallic phases form zinc corrosion products more rapidly than pure zinc when exposed to atmospheric moisture and pollutants. The resulting zinc hydroxide and zinc carbonate compounds develop characteristic brown staining appearing within months of installation.
Cosmetic Concerns:
Brown staining creates visual impression of rust or corrosion failure despite the coating continuing to provide protection. This aesthetic phenomenon prompts customer complaints and undermines confidence in galvanizing performance even though the coating functions properly.
Persistent Appearance:
Unlike wet storage stain that can be cleaned, brown staining from intermetallic corrosion products represents stable weathering that persists throughout coating life.
Economic Considerations
From the galvanizer's business perspective, excessive coating thickness increases zinc consumption and material costs. As discussed in related technical literature, zinc costs exceed typical per-pound galvanizing service pricing, meaning excess coating thickness reduces profitability.
This economic reality creates alignment between galvanizer and customer interests in limiting coating thickness—both parties benefit from avoiding excessive zinc consumption while meeting specification requirements.
Coating Growth Kinetics: Understanding the Metallurgy
Effective coating thickness control requires understanding the metallurgical mechanisms governing coating formation on reactive versus non-reactive steels:
Normal Steel Coating Formation
Low-reactivity steels (silicon content <0.03%, phosphorus <0.02%) exhibit parabolic coating growth kinetics:
Coating Thickness ∝ √(Immersion Time)
This relationship means coating thickness increases proportionally to the square root of immersion time. Doubling immersion time increases coating thickness by only 41% (√2 ≈ 1.41). The parabolic growth reflects diffusion-controlled reaction kinetics where zinc and iron atoms must diffuse through progressively thicker alloy layers to continue the reaction.
Self-Limiting Behavior:
The growing alloy layers create diffusion barriers that progressively slow reaction rates, producing inherently controlled coating thickness. Extended immersion beyond typical durations produces minimal additional thickness.
Reactive Steel Coating Formation
High-silicon steels (Sandelin range: 0.04-0.15% Si; Sebisty range: 0.13-0.28% Si) demonstrate linear coating growth kinetics:
Coating Thickness ∝ Immersion Time
Direct proportionality between thickness and time means doubling immersion duration doubles coating thickness. The linear relationship indicates reaction rates remain high throughout immersion rather than self-limiting.
Silicon Catalysis Mechanism:
Silicon accelerates zinc-iron diffusion rates, preventing the diffusion barrier effect that limits normal coating growth. The reaction maintains high velocity throughout immersion, rapidly consuming zinc and forming thick intermetallic layers.
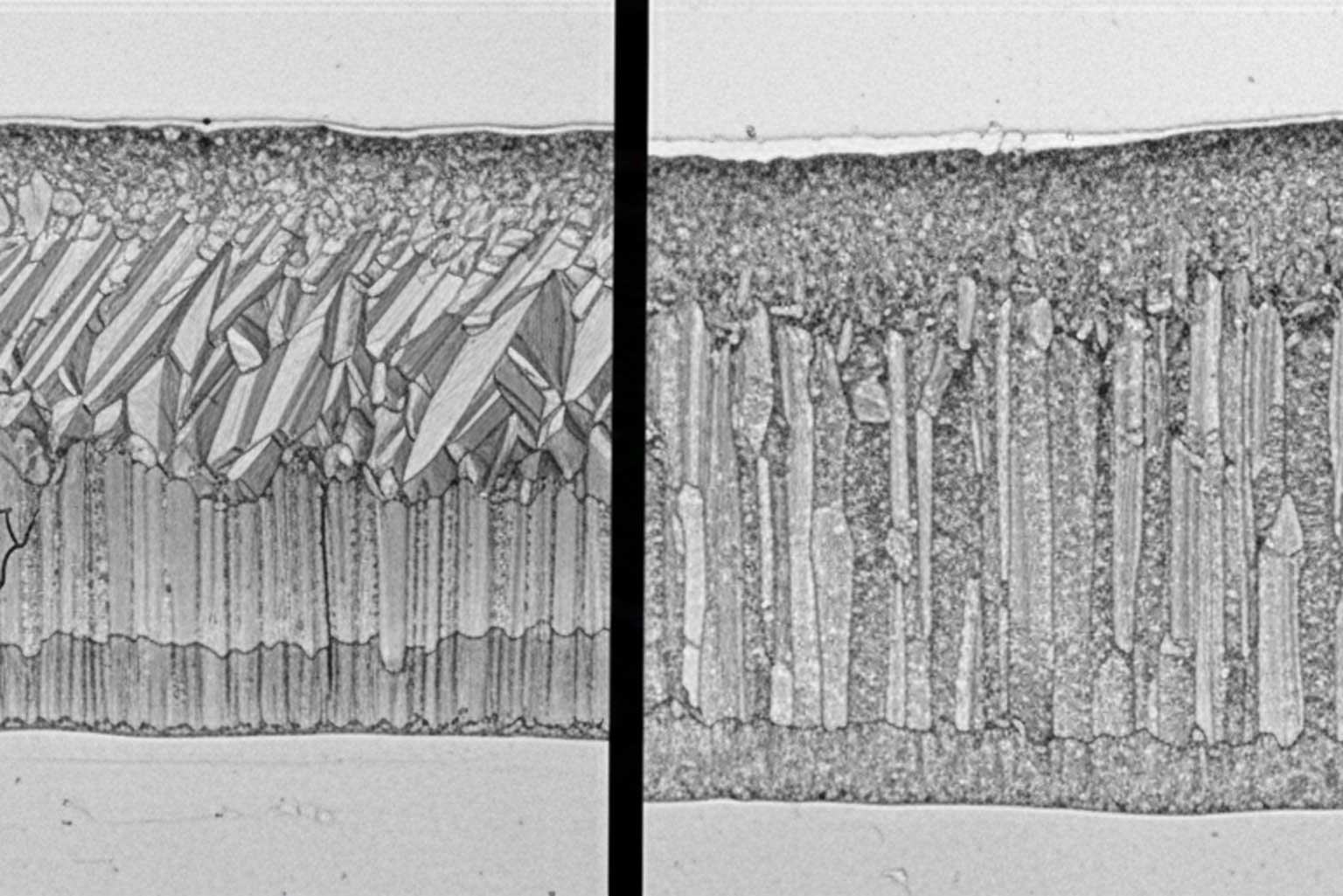
Zeta Phase Dominance:
Reactive steels produce coatings dominated by zeta phase intermetallic with characteristic columnar crystal structure. Individual zeta crystals grow perpendicular to the steel surface, reaching lengths of 100-500+ micrometers in severe cases.
Surface Profiling: Mechanical Disruption of Crystal Growth
Surface roughening before galvanizing provides the most effective and widely applicable method for limiting coating growth on reactive steels:
Crystal Growth Interference Mechanism
The zeta phase forms columnar crystals growing perpendicular to the steel substrate surface. On smooth steel surfaces, crystals grow unimpeded to substantial heights, producing thick coatings. Introducing surface roughness creates a jagged profile forcing adjacent zeta crystals to grow into one another.
Geometric Constraints:
When zeta crystals nucleate on peaks and valleys of a roughened surface:
- Crystals from adjacent peaks grow at angles converging toward one another
- Growing crystals collide and coalesce, limiting individual crystal height
- The coalescence creates broader but shorter crystal masses
- Total coating thickness decreases while maintaining continuous coverage
Effectiveness:
Surface profiling can reduce coating thickness by 20-40% compared to smooth reactive steel surfaces while maintaining adequate corrosion protection. The degree of thickness reduction correlates with surface roughness amplitude—deeper profile peaks and valleys produce greater thickness limitation.
Mechanical Surface Preparation Methods
Wheel Abrading:
Rotating abrasive wheels applied to steel surfaces create directional groove patterns:
Process Characteristics:
- Abrasive grits: 16-60 grit typical for galvanizing preparation
- Depth of profile: 2-6 mils depending on wheel pressure and grit
- Surface pattern: Parallel grooves oriented by wheel rotation
- Processing rate: Moderate—requires substantial time for large surface areas
Advantages:
- Controlled, repeatable surface profile
- Suitable for flat or gently curved surfaces
- Can be performed in fabrication shop before delivery to galvanizer
- Generates less airborne dust than blasting
Limitations:
- Difficult on complex geometries or internal surfaces
- Labor-intensive for large projects
- Equipment investment required
Abrasive Blast Cleaning:
Propelling abrasive media against steel surfaces using compressed air removes mill scale while creating surface roughness:
Process Parameters:
- Media types: Crushed steel grit, garnet, coal slag, or specialized products
- Media size: 16-30 grit typical for galvanizing preparation
- Blast pressure: 60-100 psi
- Profile depth: 2-5 mils achievable
Advantages:
- Reaches complex geometries, internal surfaces, and recessed areas
- Simultaneously removes all mill scale ensuring complete cleanliness
- Faster processing than wheel abrading for most applications
- Well-understood process with established procedures
Limitations:
- Generates substantial airborne dust requiring containment
- Media consumption and disposal costs
- Requires specialized equipment and trained operators
- Can damage thin sections if parameters not carefully controlled
Economic Considerations:
Mechanical surface preparation adds $0.50-2.00 per pound to processing costs. However, for reactive steel applications, this investment often proves economical by:
- Reducing zinc consumption (offsetting some preparation costs)
- Improving coating appearance and acceptability
- Preventing fit-up issues from excessive thickness
- Reducing coating brittleness and flaking risks
Chemical Surface Roughening: Extended Pickling
Galvanizers using sulfuric acid for chemical cleaning can roughen steel surfaces through controlled overpickling:
Extended Pickling Process:
Standard pickling removes mill scale in 5-20 minutes depending on scale thickness and acid strength. Continuing acid exposure beyond scale removal duration allows acid to attack the clean steel surface, creating microscopic roughness through:
- Preferential grain boundary dissolution
- Non-uniform attack based on crystallographic orientation
- Removal of surface layers revealing underlying texture
Duration Extension:
Extending pickling time by 50-200% beyond normal scale removal duration (an additional 5-30 minutes) produces adequate surface roughening for coating control.
Effectiveness and Limitations:
Extended pickling can reduce reactive steel coating thickness by 15-30%—less dramatic than mechanical profiling but achievable without customer-supplied pre-cleaning.
Significant Limitations:
As discussed in related technical literature, extended pickling faces substantial practical obstacles:
- Only 40% of North American galvanizers use sulfuric acid (remainder use hydrochloric acid which doesn't produce beneficial roughening)
- Many sulfuric acid operations use inhibitors preventing the surface attack needed for roughening
- Difficult to control precisely—risks excessive steel removal or insufficient roughening
- Can produce excessively rough surfaces creating fit-up or aesthetic problems
- Not applicable to assemblies with mixed steel chemistries that would experience differential attack
Zinc Bath Chemistry Modification
Alloying additions to the molten zinc bath can moderate coating formation kinetics on certain reactive steel types:
Nickel Additions
Adding nickel to zinc baths at concentrations of 0.04-0.08% effectively reduces coating thickness on Sandelin range steels (0.04-0.15% Si):
Metallurgical Mechanism:
Nickel modifies the zinc-iron reaction kinetics through several effects:
Altered Alloy Layer Formation: Nickel incorporates into the zinc-iron intermetallic structure, modifying crystal growth rates and layer formation sequences. The presence of nickel stabilizes delta phase formation relative to zeta phase, producing thinner overall coatings.
Reduced Silicon Catalysis: Nickel appears to moderate silicon's catalytic effect on zinc-iron diffusion, reducing reaction rates and coating growth velocity.
Performance Characteristics:
Effective Range: Nickel additions produce greatest benefit for steels with 0.04-0.15% silicon content—the problematic Sandelin range exhibiting unpredictable coating behavior.
Thickness Reduction: Properly optimized nickel content can reduce Sandelin steel coating thickness by 30-50% compared to unmodified zinc baths.
Appearance Improvement: Nickel-bearing baths often produce more uniform appearance with reduced mottling compared to nickel-free baths processing Sandelin steels.
Practical Implementation Challenges
Despite proven effectiveness, nickel bath additions face significant operational obstacles limiting widespread adoption:
Bath-Wide Impact:
Nickel additions affect the entire zinc bath inventory—typically 100,000-500,000 pounds of molten zinc. Once added, nickel concentration gradually increases over months as fresh zinc additions dissolve and mix. Reducing nickel levels requires partial or complete bath replacement—a major undertaking involving production shutdown and substantial cost.
Mixed Steel Processing:
Most galvanizing facilities process diverse steel types within single production runs. A bath optimized with nickel for Sandelin steel may adversely affect coating formation on:
Low-Silicon Steels: Nickel can reduce coating thickness on non-reactive low-silicon steel below specification minimums, creating coating adequacy concerns for normal material that would otherwise galvanize successfully.
Optimal Silicon Ranges: Steel with silicon content in recommended ranges (0.03-0.08% or 0.15-0.22%) may produce thinner coatings than desired when processed in nickel-bearing baths.
Economic Feasibility:
Maintaining nickel-modified baths proves economically viable only for facilities specializing in reactive steel processing with sufficiently concentrated customer base using problematic chemistries. General-purpose facilities serving diverse markets cannot justify the operational complications and risks.
Customer Communication Requirement:
Implementing nickel additions requires advance knowledge of steel chemistry—customers must provide mill test reports before production begins, enabling galvanizers to assess whether nickel baths are appropriate and whether concentration adjustment is warranted.
Alternative Bath Additives
Research has explored various other zinc bath additions for coating control:
Aluminum: Small aluminum additions (0.005-0.015%) can moderate Sandelin steel reactivity while improving coating appearance. However, aluminum also introduces complications including increased dross formation and effects on coating structure requiring careful management.
Tin: Minor tin additions affect coating formation kinetics but have limited commercial adoption due to cost and uncertain benefits across diverse steel chemistries.
Most galvanizing operations maintain relatively pure zinc baths (High Grade or Special High Grade zinc) avoiding additive complications, accepting that coating thickness control depends primarily on other methods.
Galvanizing Temperature Control
Bath temperature exerts substantial influence on zinc-iron reaction rates and coating formation:
Temperature-Reaction Rate Relationship
Like most chemical reactions, zinc-iron alloying reactions accelerate with increasing temperature following Arrhenius kinetics. Typical galvanizing temperature ranges and their effects include:
Standard Operating Range: 820-850°F (438-454°C)
This range provides optimal balance among:
- Adequate zinc fluidity for complete surface wetting
- Sufficient reaction rates ensuring coating formation
- Manageable reaction velocity avoiding excessive thickness
- Practical drainage during article withdrawal
Lower Temperature Operation: 820-825°F (438-441°C)
Operating at the lower end of acceptable ranges moderates reaction rates on reactive steels:
Benefits:
- Reduced coating growth rate—10-25% thickness reduction achievable
- Better visual monitoring of reaction completion
- Improved coating appearance in some cases
Limitations:
- Increased zinc viscosity complicates drainage, affecting coating uniformity
- Risk of incomplete wetting or cold shuts on complex geometries
- Potential for zinc solidification during processing if temperature drops excessively
- Reduced processing throughput from slower reactions requiring longer immersion
Practical Temperature Management
Dedicated Production Runs:
For large reactive steel orders, galvanizers may schedule dedicated processing shifts at reduced bath temperatures. This approach allows temperature optimization for the specific steel chemistry without compromising normal production on standard materials.
Limitations on Minimum Temperature:
Several factors establish practical lower limits on bath temperature:
Bath Size and Thermal Mass: Large kettles (50,000+ gallon capacity) exhibit thermal inertia resisting rapid temperature changes. Reducing temperature significantly requires hours of reduced heat input, limiting practical temperature adjustment for small job quantities.
Sectional Thickness: Heavy steel sections extract substantial heat from the zinc bath during immersion. Operating near minimum temperature increases risk of localized zinc solidification on thick cold sections immersed in the bath.
Zinc Freezing Risk: Zinc melts at 787°F. Operating too close to this threshold risks bath freezing during processing interruptions or equipment issues—a catastrophic operational failure requiring days to recover.
Drainage Requirements: Article withdrawal from the bath must occur while zinc remains sufficiently fluid to drain properly. Lower temperatures increase drainage time and may produce rough coating texture from impaired zinc flow.
Process Monitoring:
Experienced galvanizers can visually monitor zinc-iron reactions during immersion:
Bath Surface Indicators:
As reactive steel undergoes vigorous alloying reactions, the zinc bath surface exhibits characteristic changes—color variations, surface rippling, or appearance modifications indicating reaction intensity. At lower temperatures, these indicators appear more gradually and distinctly, allowing galvanizers to time article withdrawal precisely when reactions complete, minimizing excess coating growth.
This visual monitoring capability provides greatest advantage for reactive steels where rapid reactions make timing critical.
Immersion Time Optimization
Controlling article residence time in molten zinc offers direct coating thickness management:
Time-Thickness Relationship for Reactive Steel
The linear coating growth kinetics of reactive steels (thickness proportional to time) means immersion duration directly controls coating thickness. Reducing immersion time proportionally reduces coating thickness—a straightforward relationship enabling predictable control.
Contrast with Normal Steel:
Non-reactive steels show parabolic growth (thickness proportional to square root of time), meaning substantial time reductions produce minimal thickness decrease. For reactive steels, the linear relationship provides far more sensitive time-based control.
Minimum Immersion Requirements
While shorter immersion reduces coating thickness, several factors establish minimum times:
Complete Surface Wetting:
All steel surfaces must reach bath temperature and achieve complete molten zinc wetting. Insufficient immersion leaves areas inadequately coated or uncoated, violating specification requirements.
Thermal Equilibration:
Cold steel entering the 840°F zinc bath must absorb sufficient heat to reach reaction temperature throughout. Heavy sections require longer thermal soak times than thin sections.
Air Escape from Hollow Sections:
Tubular products and hollow assemblies require adequate time for trapped air to escape through vent holes, allowing zinc to fill internal volumes. Premature withdrawal leaves interiors uncoated.
Drainage Preparation:
Articles must remain immersed long enough that zinc wets all surfaces thoroughly, enabling proper drainage during withdrawal. Inadequate wetting produces poor drainage and rough coating texture.
Practical Time Reduction Strategies
Rapid Immersion:
Quickly lowering articles into the zinc bath minimizes the transition period where partial immersion occurs. Rapid full immersion starts the "dwell time clock" immediately, allowing shorter total cycles.
Agitation During Immersion:
Gently moving articles within the bath may accelerate thermal equilibration and surface wetting, potentially enabling shorter total immersion while maintaining complete coating coverage.
Immediate Withdrawal Upon Reaction Completion:
Attentive process monitoring allows withdrawal the moment zinc-iron reactions complete. This avoids unnecessary immersion time beyond the duration needed for adequate coating formation.
Limitations:
Aggressive time reduction risks incomplete coating coverage, particularly on:
- Complex geometries with recessed areas
- Heavy sections slow to reach reaction temperature
- Hollow articles requiring internal coating
- Articles with marginal venting and drainage design
Trial runs establish minimum practical times for specific article types and steel chemistries, balancing coating thickness control against complete coverage assurance.
Integrated Approach: Combining Control Methods
Maximum coating thickness control effectiveness typically requires combining multiple strategies:
Optimal Strategy for Known Reactive Steel:
- Pre-galvanizing surface profiling (mechanical blast or wheel abrade): 20-40% thickness reduction
- Reduced bath temperature (820-825°F): 10-25% thickness reduction
- Minimized immersion time: 10-20% thickness reduction
- Combined effect: 40-60% total thickness reduction achievable
This integrated approach transforms a 10-mil coating into a 4-6 mil coating—still exceeding typical minimums but avoiding the most severe excessive thickness problems.
The Critical Requirement: Advance Knowledge
All process control strategies require knowing steel chemistry before galvanizing begins. Without advance information indicating reactive steel, galvanizers process articles using standard parameters appropriate for normal steel. By the time excessive coating thickness becomes apparent during processing, control opportunities have passed.
Proactive Communication:
Customers should provide steel mill test reports showing silicon and phosphorus content to galvanizers days or weeks before delivery. This advance notice enables:
- Selection of appropriate control strategies
- Process parameter planning and adjustment
- Sample article trial runs if warranted
- Realistic expectation setting regarding achievable results
Realistic Expectations: Limits of Control
Despite available control methods, certain realities constrain achievable coating thickness reduction:
Metallurgical Limitations
Highly reactive steels (Sebisty range: 0.15-0.28% Si) undergo such vigorous reactions that even aggressive control measures produce 5-8 mil coatings—still substantially exceeding typical 2-3 mil normal steel results.
Complete control impossibility: The fundamental silicon catalysis mechanism cannot be entirely negated through process adjustments. Reactive steel will always produce thicker coatings than non-reactive steel of equivalent thickness and geometry.
Quality Assurance Requirements
Coating thickness control must not compromise meeting specification minimums. Galvanizers maintain conservative approaches ensuring all article areas achieve required minimums, accepting that some areas may exceed desired maximum thickness as a necessary consequence.
Economic Constraints
Extensive control measures—mechanical surface preparation, dedicated low-temperature processing runs, intensive process monitoring—increase processing costs. Cost-benefit analysis sometimes favors accepting thick coatings over expensive control measures, particularly for applications where coating thickness variation poses minimal practical concern.
Alternative Approaches
When coating thickness control proves inadequate or impractical, alternative strategies address reactive steel challenges:
Steel Chemistry Specification
The most effective approach avoids reactive chemistries entirely:
Prospective Material Selection: Specify steel with silicon content in optimal ranges (0.03-0.06% or 0.15-0.22%) per ASTM A385 recommendations when ordering material for galvanizing applications.
Mill Test Report Review: Obtain and review steel chemistry before fabrication, allowing material substitution if chemistry proves unsuitable for galvanizing.
Component Segregation
Fabricate assemblies from uniform chemistry steel rather than mixing reactive and non-reactive components. This prevents thickness variation across assemblies while simplifying processing optimization.
Alternative Corrosion Protection
For highly reactive weathering steels or silicon contents above 0.28%, consider:
- Paint systems rather than galvanizing
- Duplex systems (galvanizing plus paint) accepting thick base coatings
- Uncoated weathering steel relying on protective rust patina
Trial Processing and Sample Runs
For large production quantities of unknown or potentially reactive steel:
Sample Article Benefits
Galvanizing representative samples before production commitment enables:
Chemistry Verification: Actual coating thickness results confirm mill test report silicon content and predict production performance.
Process Optimization: Trial runs establish optimal temperature, time, and preparation parameters for the specific steel.
Appearance Assessment: Samples demonstrate actual coating appearance, enabling acceptance decisions before bulk processing.
Cost Estimation: Sample results inform accurate pricing reflecting any supplemental processing requirements.
Risk Mitigation: Problems discovered on samples prevent entire production run rejection.
Sample Specifications
Effective sample articles should:
- Represent actual production geometry and section thickness
- Come from the same steel heat as production material
- Include any critical fit-up or dimensional tolerance features
- Be processed through proposed control measure sequence
Documentation and Communication
Successful reactive steel processing requires comprehensive stakeholder communication:
Information Sharing
Fabricator to Galvanizer:
- Steel mill test reports showing chemistry
- Section thickness range and geometry complexity
- Fit-up tolerances and dimensional criticality
- Appearance requirements and acceptance standards
- Anticipated quantity and delivery schedule
Galvanizer to Customer:
- Facility capabilities for coating control
- Expected coating thickness ranges for provided chemistry
- Recommended control measures and associated costs
- Schedule implications of special processing
- Sample run recommendations for uncertain outcomes
Expectation Alignment
Clear communication establishes realistic expectations:
Achievable Goals: "For your 0.18% silicon steel, we can likely achieve 5-7 mil coating using blast preparation and reduced temperature processing."
Uncertain Outcomes: "This chemistry sometimes produces 8-12 mil coatings. We recommend sample runs before committing the full production quantity."
Economic Reality: "Coating control measures will add $X per pound to processing costs. For this application, accepting thick coatings may prove more economical than extensive control efforts."
While galvanizers possess several methods for limiting coating growth on reactive steels—including mechanical surface profiling, extended chemical pickling, nickel bath additions, reduced galvanizing temperature, and minimized immersion time—none provide complete control matching the inherently controlled coating formation on non-reactive steels. Surface profiling through abrasive blasting or wheel abrading offers the most effective and widely applicable approach, reducing coating thickness by 20-40% through geometric disruption of columnar zeta crystal growth. Bath temperature reduction and immersion time minimization provide supplemental control, collectively achieving 40-60% thickness reduction when combined with surface preparation. However, highly reactive Sebisty range steels (0.13-0.28% silicon) produce thick coatings despite aggressive control measures due to fundamental silicon catalysis effects that cannot be entirely negated. All control strategies require advance knowledge of steel chemistry through mill test report review, enabling proactive process planning and realistic expectation setting. The most effective approach remains prospective steel specification avoiding reactive chemistries per ASTM A385 recommendations, with coating control measures serving as mitigation for situations where reactive steel is unavoidable. When control measures prove inadequate or economically impractical, alternative strategies including material substitution, component segregation, or alternative corrosion protection methods warrant consideration. Successful reactive steel management demands transparent communication among designers, fabricators, and galvanizers, with trial sample processing recommended for large production quantities to verify performance before bulk commitment. To learn more, see the original AGA resource on Controlling Coating Thickness.